Integrated, biological diagnostics on a molecular level
Fraunhofer CMI and their research collaborators from Boston University developed a fully integrated lab-on-a-chip and associated instrument for the detection of bacteria from liquid samples in this prototyping project in biomedical engineering.
Objective
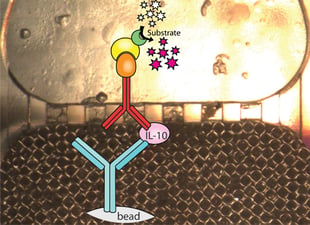
The major challenges of point-of-care clinical diagnostics are sample preparation and detection accuracy. The field of clinical diagnostics is moving toward molecular testing, such as polymerase chain reaction (PCR), which is the most sensitive and specific methodology currently available. On the other hand, PCR suffers from labor intensive sample preparation to isolate nucleic acids and physical separation requirements in a laboratory to minimize sample contamination. Existing point-of-care diagnostics use antibody-based immuno-detection, which enables simple test protocols but lacks the sensitivity and specificity of molecular techniques. To address this challenge, Fraunhofer CMI aimed to design an integrated lab-on-a-chip consumable and a prototype instrument.
Methodology
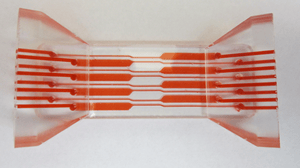
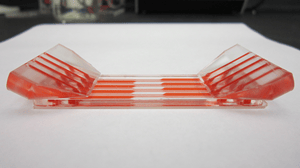
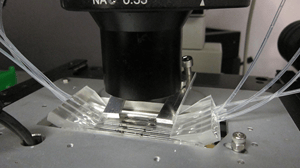
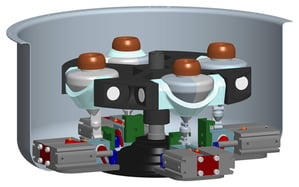
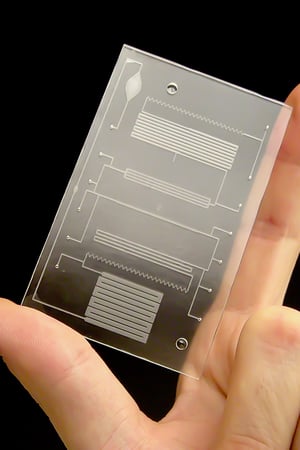
The system conducts bacterial lysis, nucleic acid isolation and concentration, polymerase chain reaction (PCR), and end-point fluorescent detection. Approximately the size of a credit card, the cost of the plastic, microfluidic chip is minimized by keeping all active components in the instrumentation (valves, heating units, optics, etc.) and by having a simple, planar layout. A novel porous polymer monolith (PPM) embedded with silica is incorporated onto the chip to lyse bacteria and isolate nucleic acids from clinical samples. The prototype instrument automates fluidic handling, thermal control, and optical detection using a unique, valveless switching fluidic control system.
Results
Integrated chip design includes bacterial and mammalian cell lysis, mixing reagents, isolation and concentration of nucleic acids, PCR, and fluorescence detection
Automation of fluidics, thermal cycling, and optical detection
Low-cost manufacturing design
Bacterial and viral detections in a point-of-care setting were equivalent in sensitivity and specificity to a hospital's clinical laboratory.
Collaborators
Department of Biomedical Engineering, Boston University, Boston, MA
Funding
This project was funded by the BU-Fraunhofer Alliance for Medical Devices, Instrumentation, and Diagnostics.