Objective
Tissue homogenization is a critical step for many research and industrial processes. From grinding down food products to search for microbial contamination or by generating single cell suspensions for downstream immunological assays or ethically sourced mesenchymal stem cells, this labor intensive task slows researchers down. Although techniques exist for such tissue homogenization, few options are available for processing large sample arrays in parallel. Standard techniques require skilled users, are often unsuitable for solid tissue samples, or prove too costly and time-intensive.
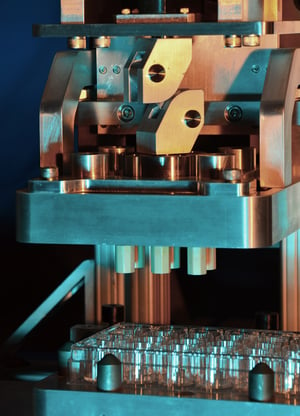
To address these issues, Fraunhofer USA CMI developed a prototype instrument capable of quickly homogenizing an array of unique tissue samples directly in a microtiter plate. No special training was necessary to achieve uniform, repeatable results—the process accommodated semi- or full automation. The system was easy to clean and sterilize, had configurable speed and force to control shear and to minimize heating, and was useful for a wide range of sample sizes.
The design utilized a novel linkage mechanism that both transmitted torque from the rotary motor to the pestle plate and varied the orbital radius of the pestles. The multi-function linkage prevented sample smearing against the microtiter plate walls and reduced the risk of dripping and cross-contamination. By eliminating the need for an independent actuator to control the orbit radius, the machine’s complexity and cost were decreased.
This tissue homogenizer can be flexibly incorporated into existing workflows such as the large automated stem cell factory located at Fraunhofer IPT. Due to its compact engineering, it can also efficiently function as a stand-alone instrument for on-demand tissue processing.
Results
- Designed technique to rapidly homogenize samples in a microtiter plate
- Required no special training to achieve uniform, repeatable results
- Controlled shear and heating by adjusting motor speed and force
- Created multi-function linkage to transmit torque from rotary motor to pestle plate and to vary pestle orbital radius
- Achieved low-cost scalability and compatibility with downstream biological analysis
- Accommodated wide range of sample sizes and consistencies
- Produced more viable cells than manual methods in a fraction of the time (1 min v. 30 min)
Collaborators
Fraunhofer IPT, Aachen, Germany
Funding
Built for two separate biotechnology firms in the U.S.